A Phosphonates Primer in Cooling Water Treatment
Choose for a Reason
Stephen M. Kessler
Technical Sales Leader
Deposition in cooling systems is an insidious problem that can lead to decreased efficiency, unscheduled down-time, and elevated operational/maintenance costs. Deposits can especially accumulate in low circulation areas, becoming immobilized and building up on heat exchanger surfaces. Once a scale layer is formed, it will continue to get thicker unless treated, eventually blocking flow, and decreasing productivity. Therefore, an effective water treatment program must control scale, corrosion, particulates, and biological growth.
Cooling water treatments typically can be divided into two groups, i.e. “Stabilized Phosphate (SP)” and “Alkaline Treatments (AT)”. SP programs contain high dosages of inorganic phosphate for mild steel corrosion control, lower dosages of phosphonate for calcium carbonate deposit control, sulfonated acrylic acid copolymer/terpolymers for phosphate inhibition/particulate dispersion, and an azole for yellow metal corrosion control. These typically operate in the 7.0 to 8.0 pH regime.
AT programs greatly reduce and/or eliminate inorganic phosphate, utilize higher dosages of phosphonate for both carbonate and corrosion control, polymers for both phosphate/phosphonate deposit control, and azole for yellow metal protection. These typically operate in the 8.0 to 9.0 range. This Brief will focus on current AT technology utilizing three common phosphonates operating at highly buffered conditions.
Other considerations would be phosphonate performance in the presence of iron in your system as well as the temperatures of operation.
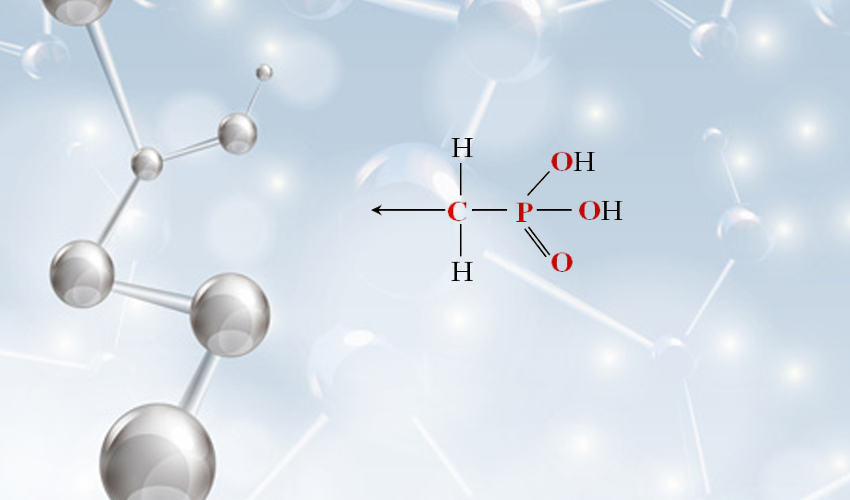
Scale formation is prevented by using threshold inhibitors, i.e. compounds that adsorb onto crystal growth sites and inhibit or alter crystal growth/morphology. They are utilized at small amounts not even approaching concentrations required for sequestration for effective calcium carbonate control. Phosphonates are Threshold Inhibitors.
Three commonly used phosphonates are HEDP (1-Hydroxyethlidine 1,1-diphoshonic acid), PBTC (2-Phosphonobutane 1,2,4-tricarboxylic acid), and HPA (Hydroxyl phosphonocarboxylic acid). Use of these can extend cooling water operation to up to a Langelier’s Saturation Index (LSI) of 2.8+. Additionally, effective corrosion control is also attained with these chemistries. Both PBTC and HEDP provide protection by the formation of a controlled calcium complex (cathodic protection) on the metal surface. Whereas, HPA is a bit more unique in that it does not rely on this complex, but rather by reacting with the corrosion process at the metal surface (anodic protection). When designing a treatment program for your system, each has benefits which may be important for your application.
For example, HPA is preferred under soft water conditions where calcium concentration may be lacking for use of PBTC or HEDP. Mild steel corrosion control comparison is best utilizing HPA. Halogen resistance of these molecules in the presence of chlorine should also be considered. PBTC is most chlorine resistant and performs best with oxidizing biocides. Under elevated free chlorine levels, PBTC performs better than HEDP which performs better than HPA.
Other considerations would be phosphonate performance in the presence of iron in your system as well as the temperatures of operation. All these parameters influence the choice of which phosphonate to use.
At Delta, we will review, customize, and design a superior cooling water treatment using the preferred phosphonate or phosphonate blend package to address your specific water treatment challenge(s). This will go a long way to ensure your system is performing most efficiently and providing you an extended operational life at reduced energy costs.
In general, phosphonates have the same function in cooling formulations, the degrees of efficacy for each just varying under different water conditions. This is the reason they must be chosen carefully for your application.