Performance of Once-Through
Cooling Water Treatments
Stephen M. Kessler
Technical Sales Leader
Once-through cooling water is prevalent throughout many industries. These streams are known in the pulp and paper industry as "mill supply waters", as "service water" in the power industry, and as "once-through waters" in the chemical and hydrocarbon process industries. Large volumes of water are typically drawn from a nearby well, river, or lake for most of the applications and then discharged to an aqueous stream. Outside of mechanical screening to remove large contaminants, only relatively low treatment concentrations can be applied to those streams because of costs associated with treating large volumes of water.
A mill supply treatment typically consists of both corrosion and deposit control agents as well as some type of microbiological (MB) control protocol. Threshold concentrations of inorganic phosphate are typically applied in combination with zinc, polymer, and/or hydroxylated carboxylic acids (HCAs). Protection of carbon steel is the main aim of these programs because it is the predominate metallurgy for most mill supply transfer piping. While copper-based alloys may be a minor component in these loops, it is not cost effective to incorporate azoles for protection of these alloys. Zinc discharge to rivers and streams may also be regulated in some areas but concentrations as low as 0.1 to 0.3 ppm Zn can contribute significant corrosion inhibition to any treatment matrix. If zinc cannot be used, there are several HCA's which offer enhanced corrosion inhibition and are an effective substitute for that cation.

Typically, 0.5 to 3 ppm inorganic phosphate, 0.1 to 1.0 ppm Zn, 0.5 to 2.0 ppm polymer and/or 0.25 ppm to 1.5 ppm HCA are applied to mill supply process waters. While untreated streams can yield carbon steel corrosion rates in excess of 50 mpy, phosphate/polymer blends with either Zn or HCA can provide corrosion rates of 5 to 10 mpy. Blends of only phosphate and polymer will provide corrosion rates in the 10 to 15 mpy range, with moderate pitting.
The most common corrosion mechanism found mill supply applications can be depicted by this classic corrosion cell.
While orthophosphate is the most economical inorganic phosphate, it does not possess the sequestering ability of pyrophosphate.
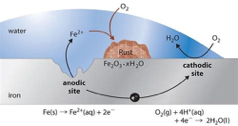 Metal loss occurs at the anode with dissolution of the base metal as electrons flow from anode to cathode. Besides the detrimental effect of general metal loss, this process can also lead to corrosion by-product (iron oxide and hydroxides) accumulation, leading to a reduction in heat transfer and flow, resulting in higher operating costs. Inorganic phosphates, zinc, and/or HCA are commonly used for corrosion control in once-through systems. Their performance is based on minimizing the cathodic reaction. While orthophosphate is the most economical inorganic phosphate, it does not possess the sequestering ability of pyrophosphate. Pyrophosphate is exceptionally effective at minimizing tuberculation, sequestering iron and manganese, and aiding in scale control. This mechanism of cation complexation is particularly critical to the pulp and paper industry where ineffective iron or manganese stabilization can adversely affect paper brightness. Polymer inclusion in these treatment blends help to keep all solids dispersed with minimal accumulation through the cooling loop.
Metal loss occurs at the anode with dissolution of the base metal as electrons flow from anode to cathode. Besides the detrimental effect of general metal loss, this process can also lead to corrosion by-product (iron oxide and hydroxides) accumulation, leading to a reduction in heat transfer and flow, resulting in higher operating costs. Inorganic phosphates, zinc, and/or HCA are commonly used for corrosion control in once-through systems. Their performance is based on minimizing the cathodic reaction. While orthophosphate is the most economical inorganic phosphate, it does not possess the sequestering ability of pyrophosphate. Pyrophosphate is exceptionally effective at minimizing tuberculation, sequestering iron and manganese, and aiding in scale control. This mechanism of cation complexation is particularly critical to the pulp and paper industry where ineffective iron or manganese stabilization can adversely affect paper brightness. Polymer inclusion in these treatment blends help to keep all solids dispersed with minimal accumulation through the cooling loop.
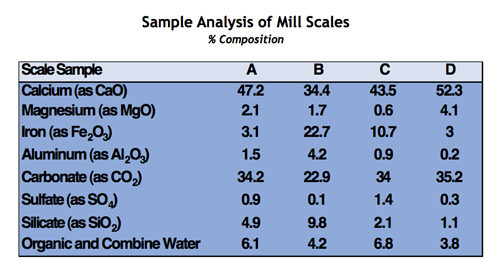
The control of general scale formation is critical in once-through cooling loops in order to ensure the efficient operation of associated heat transfer equipment and also to minimize under-deposit corrosion. Scales typically found in these systems contain mainly calcium carbonate and iron corrosion byproducts. Silt and organic microbiological matter can also be present to a lesser extent. The table below depicts analytical results of typical deposits found in once through applications.
While water softening would alleviate hardness scale, this is not economically feasible.
Therefore, CaCO3 scale formation remains a concern, especially in hotter systems, due to its inverse solubility. This problem can be controlled, however, with the use of deposit control agents such as polyphosphates, organic phosphates, and polymers. These materials prevent scale formation by either sequestering specific scale-forming cations, or by modifying the crystal growth patterns of the inorganic salts. This allows them to exist in a state of supersaturation rather than precipitate out in the bulk phase. Treatment concentrations ranging between 0.5 and 2.0 ppm can effectively control most scales in once-through systems. Deposition of iron, present as a result of corrosion or entering from wells or surface water, is also of concern. In addition, soluble manganese can be present at concentrations of up to 1 ppm, depending on the makeup source for the mill water. Once oxidized, manganese can also cause deposition leading to under-deposit corrosion and pitting. This can be effectively controlled with the use of either pyrophosphate or hydroxyethylidene diphosphonic acid (HEDP). Pyrophosphate is further enhanced when combined with certain polymer and copolymers.
Finally, silt and MB-related deposition must be addressed. Polymeric materials can handle most solids problems by altering the surface charge characteristics of the particles, keeping them suspended and flowing through the system. Typical polymer dosages of between 0.5 and 1 ppm are effective for suspended solids control. MB problems are typically addressed by halogenating (chlorine or bromine) the makeup source prior to use. Halogen feed for effective MB control is very application-dependent and varies according to the nature of the system and water source.
Please feel free to contact Delta Water Management Group regarding any once-through application at your facility. We will assess your current water quality and design the most cost-effective treatment to improve your operational efficiency while also extending equipment life.